[This is one of a series of posts we plan on the second wave in India. Previous posts have presented (1) an overview and discussion of the Amravati outbreak and (2) the behavioral change theory. Here we discuss the role that new variants could be playing in wave. We conclude that the two factors that together are most likely to have caused the second wave are a large pocket of susceptible persons that escaped the first wave and new variants of SARS-CoV-2 that are more contagious that earlier variants.]
A common explanation for the second wave (Figure 1) is new genetic variants. Some are from the UK, South Africa and Brazil. But one mutation, B.1.617, has originated in India. It has three mutations, L452R, E484Q, and P681R, thought to increase transmissibility. While these new variants are a necessary explanation for the second wave, they are not sufficient. Indeed, they are only a complete explanation in combination with behavior -- but in a surprising way.
Figure 1. Cases and deaths in the first and second waves in India.
Notes. Data from covid19india.org.
A framework to interpret data
We start with a simple framework to help us translate the data on the first and second waves into conclusions about the role of new variants (and behavioral change). This framework divides the population into two groups and the virus into two classes of strains. One group of people, the immune population, includes those who were infected or vaccinated before the second wave. (Most members were infected, as only 1.8% of the population was vaccinated by March.) The second group, the susceptible population, is everyone else. We divide variants into the original strain(s) of SARS-CoV-2 and into the new variants, including foreign and the Indian strains. The different strains have potentially different effects on the different populations.
Table 1 sets forth our framework. In this post we are interested in using the framework to understand the risk of infection holding (personal) behavior constant. Risk is usually defined as the probability of infection. That in turn is has three parts:
The probability of interacting with another person (i.e., activity level)
The probability that person is infected
The probability of infection given meeting someone who is infected (i.e., transmissibility)
In the last post we examined behavior, which primarily affects the probability of meeting another person. In this post we study new genetic variants of SARS-CoV-2, which -- relative to behavior -- affect factors two and three more. We will call these two factors the “risk of interaction”.
Table 1. Framework applied to the risk of interaction, defined as the probability of infection given a level of activity.
To understand the effect of different viral strains on the risk of interaction to susceptibles, it helps to understand the effects of natural selection. Viruses mutate constantly. A mutation persists only if it confers some advantage in viral replication. The mere existence of the new strains tell us that they must increase “fitness” in this way. Among the susceptibles, the old variants cannot be any more risky than they were last year: the rate of replication cannot change unless there is a mutation (cell A). The new variants, however, must be more risky (cell B).
To understand the effect of the virus on the immune population, we need to understand how the immune system works and, in particular, how it is helpfully “senile”.
How the immune system and vaccines function
When the body is infected with a new virus, unlike anything the body has seen before, its immune system is slow to recognize and attack it. The immune system sends scouts (antibodies) to find the infection. Some fail because they don’t know how to spot (or attach to) the virus, but some ultimately succeed. Once they attach to the virus, they signal help (e.g., white blood cells) to come and destroy infected cells, stopping the virus from replicating. This process is slow because the body does not recognize a new virus. But after the infection has been cleared, certain cells (t-cells) of the immune system remember what the virus looks like (i.e., its strain) so that, if it returns, the immune system can more quickly identify, attack and clear it. A person that is immune can be reinfected, but she will clear the infection faster; so she will experience fewer health impacts and she will be infectious to other people for less time.
A vaccine tries to short-cut the process of generating immune memory by introducing, usually via injection, bits of the infection into the body. The bits are weakened or inactive versions of the virus, or even just useless fragments of the virus, hoping to induce an attack by the immune system without the harm from viral replication. The end result is, ideally, immune memory of the viral strain mimicked by the vaccine.
What is surprising about the immune system is that it is senile -- and that is a good thing. The immune system remembers sort of what the virus (or virus fragments in a vaccine) looks like, but not exactly what it looks like. Its antibodies may identify parts of the virus but not all of it. This is helpful because viruses mutate all the time. If the immune system could only identify the exact strain that invaded the body, even a small mutation would throw the system off and it would have to go through a slow learning process again. But its senility means that it will mistake strains that are only somewhat different for the original strain, and still attack it quickly. (This is called cross-reactivity.) The cross-reactive attack may be somewhat less effective than if the identical strain returned, but the attack could be good enough.
Back to our framework
There are two practical implications of this model of the immune system for understanding the effects of different strains on the immune population. First, old variants are likely to post little risk for those who are immune (cell C). While immunity does not stop reinfection, the risk of spread is lower. Immune people likely hang out with others that are immune, in part because they had the infection and either got it or spread it to their regular contacts. That means that, going forward, if their likely contacts do catch the infection they are likely to be contagious for less time.1
How do we know this? We do not have a lot of direct evidence on how protective natural immunity -- immunity obtained via prior infection -- is against different strains. However, we have useful indirect evidence from studies of how protective vaccines are against different strains. Recall that vaccines trigger the same mechanism to generate immune memory -- and thus protection -- as viruses do. What evidence we have on vaccines so far suggests that they are very effective against new strains. While they do reduce harm, e.g., death, by 70-90%, that is not what we are talking about. What is more relevant for risk of infection is the extent to which they reduce infection. Studies suggest those who are vaccinated have lower rates of infection and transmission and that communities, such as Israel, where vaccination is widespread, infection rates have fallen dramatically.
Second, although the naturally immune were likely initially infected with the old variants (and the few who were inoculated took vaccines targeting those same variants), the immune population is likely to have some protection -- or cross-reactivity -- to the new variants because the immune system is senile.
How much protection? One piece of evidence is that those with prior infection showed neutralizing antibody response to the South African variant. Another piece of evidence again comes from those who were vaccinated. Individuals vaccinated with the Pfizer vaccine show neutralizing antibodies to the UK strain. Corroborating evidence comes from those vaccinated in Israel. Vaccination with the Pfizer vaccine was associated with lower infection, even though those vaccinated were exposed to the South Africa variant. (Although variants were more common among those that are vaccinated than those that are not, that reflects the fact that those vaccinated are more protected against old variants than new variants.) There is even preliminary evidence that the Covaxin vaccine, on the two vaccines initially approved in India, generates neutralizing antibodies to the Indian B.1.617 variant.
These results suggest that those who are immune have lower rates of infection from the new variants than the susceptible. To be fair, however, it does not tell us whether the rates of infection with new strains amongst the vaccinated (cell D) are higher or lower than the rate of infection with the old strain amongst the susceptibles (cell A).
A combination of susceptibles and new variants are the dominant cause of the second wave
If we step back and combine our framework with the data we see in the second wave, we are inclined to believe that the second wave is primarily due to a combination of a large pocket of susceptibles and new variants (cell B).
First, there is evidence that individuals who escaped the first wave are the bulk of people being infected in the second wave. The first wave largely infected poorer populations. Just before the peak of the first wave, one of us (Malani) was involved in two sero-surveys in Mumbai, in July and August 2020. Both found that the prevalence of antibodies to old variants was about 3 times higher in slums than in non-slums. Consistent with this, data on mobility in Mumbai obtained from smartphone location data suggests that slum residents were quicker to return to work after the lockdown and before the peak of the first wave than were non-slum residents (Figure 2). This is corroborated in the rest of the country by analysis of monthly income patterns across income quartiles. These show that individuals in the highest income quartiles were slower to return to work after the lockdown (Figure 3). Finally, surveillance during the second wave confirms that it is disproportionately composed of individuals who are from non-slums (Figure 4, from Murad Banaji). Our conversations with people who have looked at the official data from Mumbai confirm this pattern.
Figure 2. Mobility in slums and non-slums of Mumbai through the peak of the first wave.
Notes. Mobility in the left (right) figure is measured by the number of unique locations visited (trips taken) by slum and non-slum residents. Home location (locations visited) is measured on an Uber H12 (H10) partition of Mumbai and its suburbs. Details in Sheng, Malani, Goel and Botla (forthcoming Journal of Urban Economics, 2021).
Figure 3. Income relative to average 2019 income over 2019 and 2020, across income quartiles (4th = highest income quartile).
Notes. Income is individual income for respondents in the Centre for Monitoring Indian Economy’s Consumer Pyramids Household Survey. Quartiles are defined by average 2019 household income. Analysis is from Gupta, Malani and Woda (2021)
Figure 4. Estimates of the daily cases from slum and non-slum areas in Mumbai.
Note. This figure is from Murad Banaji can can be found here.
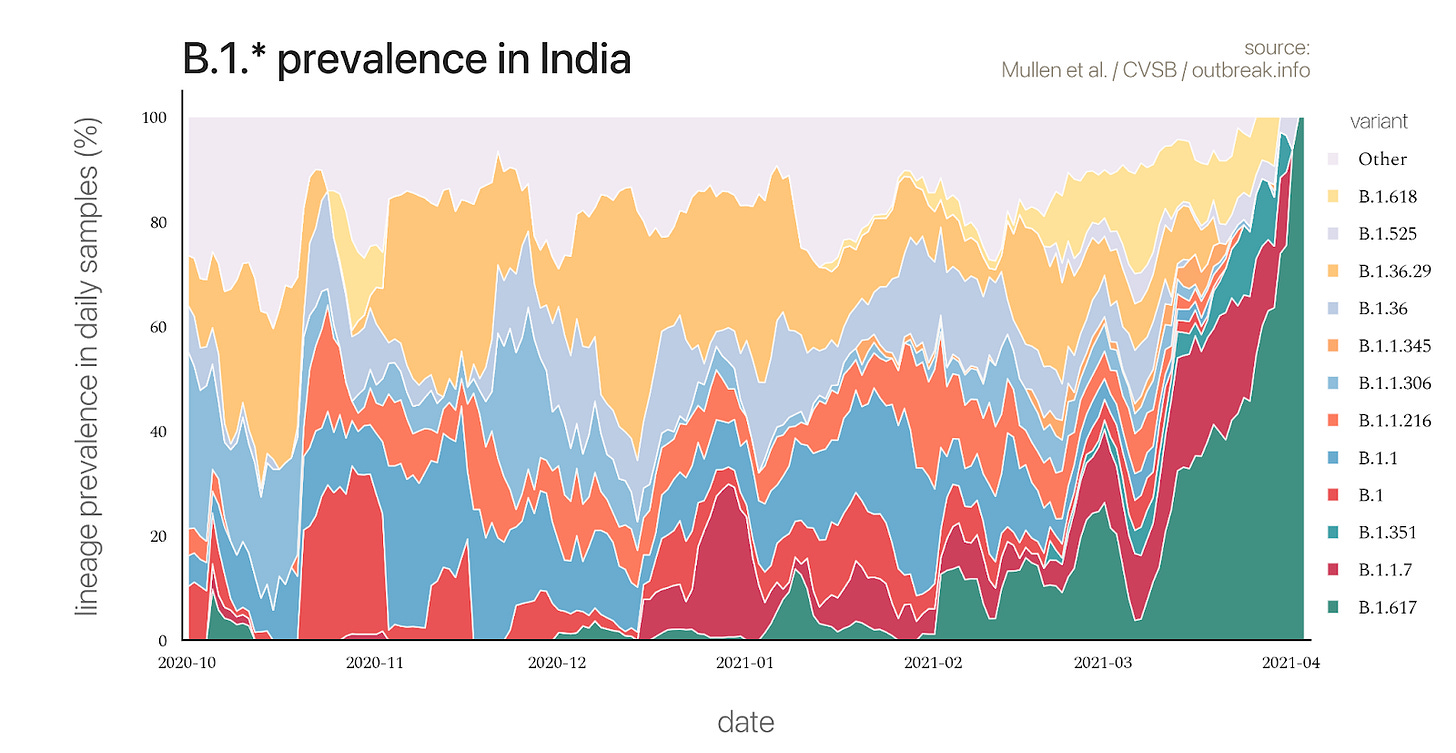
Second, the sequencing of COVID-positive swab samples during the second wave and the rate at which infections exploded2 suggests that new variants are a critical component. The new variants are a large share of the viral specimens sequenced in the second wave (Figure 5). This is direct evidence that new variants play a major role in the second wave. Moreover, the second wave hit much faster and reached a higher peak than the first wave (Figure 1). This implies a higher reproductive rate; we did not see a similar reproductive rate last year when older strains were circulating and susceptible individuals had similar levels of activity.
Susceptibles combined with old variants cannot explain the second wave. As explained earlier, there is no reason to suspect that the old variants were more contagious among susceptibles in 2021 than in 2020. Indeed, if they were, the susceptibles in the second wave would have been infected in the first wave. Nor can new variants among the previously immune explain the second wave. Susceptibles form a disproportionate share of the new infections.
Figure 5. Frequency different COVID mutations in India.
The role of behavior
In a previous post we dismissed an increase in activity as a cause of the second wave. We noted that mobility did not increase sharply before the second wave hit. However, our conclusion that the combination of susceptibles and new variants are the biggest causes of the second wave does not mean behavior plays no role. It is likely that new variants had a larger effect than it otherwise would have because susceptibles have gradually been increasing their level of activity over time. More importantly, our conclusion points to another behavior that may be responsible for the size of this second wave: early social distancing.
The behavior that may be responsible for the second wave is that certain populations effectively socially distanced and escaped the first wave. If the epidemic somehow petered out after the first wave, or if remaining susceptibles were vaccinated before a second wave could strike, then there may not have been a second wave. But absent those fortunate turn of events, those who escaped the first wave were just field for the second wave. We are seeing this in other countries, including China and New Zealand, that successfully used non-pharmaceutical interventions to mitigate the effect of COVID in 2020. These countries are now facing ongoing risk because they cannot vaccinate faster than the epidemic reaches even their protected populations.
This is not an argument against social distancing. However, it is a reminder that social distancing, without vaccination, may only delay the inevitable.
Implications for policy and the future
Our discussion about the likely causes of the second wave suggests three interesting predictions going forward. First, the second wave will likely end nearly as fast as it arrived. A high rate of reproduction means the epidemic will ravage the remaining susceptible population more quickly. It is possible that lockdowns will bend the curve so it lasts longer. We are now seeing some of this in places like Amravati district, which locked down and then saw a return of cases when lockdown was relaxed (Figure 6). But, in general, we have seen, even with targeting lockdowns, infection waves seem symmetric across countries. Hence, a sharp rise in the aggregate curve probably portends a sharp fall in such cases.
Second, the size of the second wave, as measured by the number of people infected for the first time3 during it, is likely equal to the number of people who remained susceptible after the first wave. It is hard to imagine that any large portion of the Indian population will be able to maintain adequate social distancing -- and thus remain susceptible -- beyond May or June. (However, we would be happy if they did.) If we think that half the population was immune after the first wave, a not unreasonable estimate based on prior serological surveys and projections therefrom, then the second wave will be about the same size as the first wave.
Third, just as the first wave infected enough people and lingered long enough to generate the B.1.617 variant, the second wave and continual lingering of that variant after the wave due to slow vaccination creates the risk of yet another variant. If the next, new Indian variant keeps drifting away from the initial COVID variants, then the third wave is more likely to after those infected in the first wave than those in the second wave, e.g., more likely to hit slums than non-slums. The best way to mitigate that risk is to redouble efforts at vaccination. Moreover, India may want to consider vaccinating against the B.1.617 strain, assuming that is possible.
Conclusions
We think that a combination of a large pocket of susceptible persons and new variants are probably the largest drivers of the second wave. This does not mean that other cells (A, C, and D) play no role; just that they play a lesser role. Moreover, it does not mean that behavior did not matter. But if you had to pick a more important driver, we would say it is cell B, the combination of susceptibles and new variants. The fact that mobility was higher at the start of the second wave than at any time after last year’s national lockdown certainly helped fan the flames. We suspect, however, that the second wave might have happened even if mobility plateaued in December. We don’t think it would have been as serious without a large number of susceptibles (because of the value of immunity) and new variants that are more contagious.
Figure 6. Mobility over time in India and Maharashtra.
This is assuming people who are immune are going out only as much as people who are not. Since immune people are probably meeting a lot more people than those who are not, it is ambiguous whether susceptibles or immune have the higher risk, allowing behavior to vary. Of course, the fact that we find that the susceptibles are a higher portion of those infected suggests that, despite less activity, the susceptibles have higher risk. This reinforces our conclusion that combination of susceptibles and new variants are most responsible for the second wave.
To see this, consider Figure 6. As the second wave hit, mobility cratered. If we take a look at a week or two into that drop we see that Rt is high despite the fall in mobility. Moreover, if we move left in that curve we can see similar levels of mobility in December or January, a time when old variants were more common. Yet we saw Rt hover around 1 at that time, not at 2 or so, as we see now.
It is possible the second wave could be larger than the number of susceptibles after the first wave because of the risk of reinfections among the immune. If the level of reinfection is low or if reinfections generate few symptomatic cases, we will not observe those numbers in infection counts as there is little population-level testing or testing of those who are asymptomatic.










I find this to be one of the most cogent explanation of situation unfolding in India. Why certain and large section of population remained unexposed in first wave and thus susceptible is unanswered. The fading of first wave is puzzling. About sero-prevalance, will antibodies be present even when received quantity of virus was very low? Is it possible that much of the population received virus strain of first wave but in varying proportion? Thus the susceptible are those whose virus encounter was too brief. The other explanation would be post-national lockdown mobility (from 1 June 20) was such that it created pockets of susceptible population, but it seems far fetched considering (casual empiricism) level of activity during year end (Nov and Dec). In any case, difference in properties of virus strains should be focus of explanation, as you have. Thank you.